welcome to CARTI clinical research
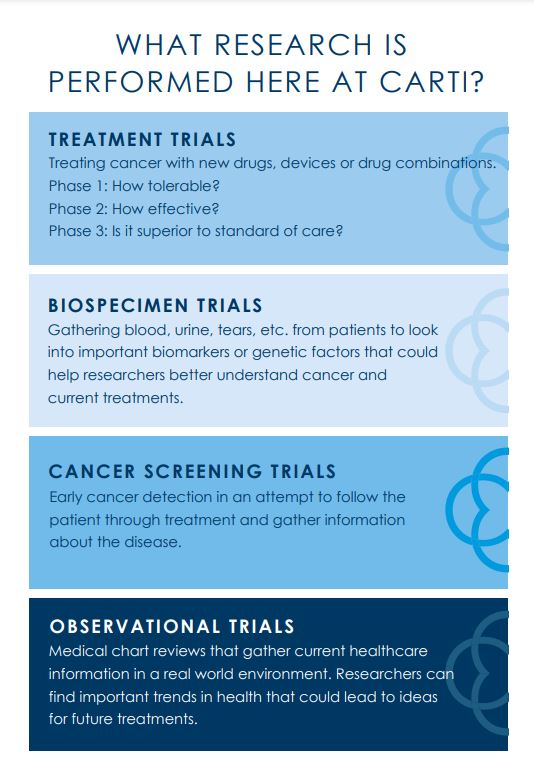
Our research team is currently working with pharmaceutical companies on over 35 clinical trials to bring advancement to oncology medicine. Clinical trials examine the safety and efficacy of new treatments, reduce the time between treatment and response evaluation, improve supportive care medications, monitor treatments in the real world setting and provide biological samples to develop tests for early cancer detection. Here at CARTI we are proud to offer front-line research to our patients. Our goal is to work toward innovation and improvement in standard of care. With a mission of providing the most leading-edge cancer care available, CARTI’s medical team actively participates in research projects that will impact the treatment options we are able to provide our current and future patients.
how do I participate in a clinical trial?
The purpose of our research is to engage in research-driven patient care by offering the most effective new treatment options in order to extend survival, improve quality of life and advance the knowledge of cancer treatment. If you are interested in participating in a clinical trial, ask your physician if you qualify. To make our clinical trials available to all CARTI patients, all of our physicians serve as sub-investigators; this means that your treating physician can enroll you onto a clinical trial if you are eligible. Please refer to the list below to view our active studies!
Participation in research is always voluntary. For further information on clinical trials, please visit http://www.clinicaltrials.gov.
If you have any additional questions for or about CARTI’s Research Team, please call 501.906.4199 or email Research@CARTI.com.
BREAST CANCER TRIALS

Adjuvant WIDER; CLEE011O12011
Sponsor: Novartis AG
Principal Investigator: Dr. Sam Makhoul
A Phase IIIb Study to Characterize the Efficacy and Safety of Adjuvant Ribociclib Plus Endocrine Therapy In a Close-to Clinical Practice Patient Population With HR+ HER2− Early Breast Cancer
ClinicalTrials.gov ID: NCT05827081

Fourlight-03; C4391024
Sponsor: Pfizer, Inc.
Principal Investigator: Dr. David Kuperman
A Study to Learn About the Study Medicine Called PF-07220060 in Combination With Letrozole in People Over 18 Years of Age With HR-Positive, HER2-Negative Advanced/Metastatic Breast Cancer who Have not Received any Prior Systemic Anticancer Treatment for Advanced/Metastatic Disease
Clinical Trials.gov ID: NCT06760637

TROPION-SWISH; D926UL00001
Sponsor: AstraZeneca Pharmaceutical LP
Principal Investigator: Dr. David Kuperman
An open-label, single-arm study of prophylaxis of Datopotamab deruxtecan (Dato-DXd) -related stomatitis in eligible patients with metastatic or inoperable locally recurrent breast cancer or locally advanced or metastatic epidermal growth factor receptor-mutated non-small cell lung cancer.
LUNG CANCER TRIALS

TROPION-LUNG 10; D7632C00001
Sponsor: AstraZeneca Pharmaceutical LP
Principal Investigator: Dr. Ryan Hall
Phase III, Open-label, Study of First-line Dato-DXd in Combination With Rilvegostomig for Advanced Non-squamous NSCLC With High PD-L1 Expression (TC ≥ 50%) and Without Actionable Genomic Alterations
ClinicalTrials.gov ID: NCT06357533

MERCK MK2870-023
Sponsor: Merck & Co.
Principal Investigator: Dr. Ryan Hall
Pembrolizumab With or Without Maintenance Sacituzumab Tirumotecan (Sac-TMT; MK-2870) in Metastatic Squamous Non-small Cell Lung Cancer (NSCLC)
ClinicalTrials.gov ID: NCT06422143

TROPION-SWISH; D926UL00001
Sponsor: AstraZeneca Pharmaceutical LP
Principal Investigator: Dr. David Kuperman
An open-label, single-arm study of prophylaxis of Datopotamab deruxtecan (Dato-DXd) -related stomatitis in eligible patients with metastatic or inoperable locally recurrent breast cancer or locally advanced or metastatic epidermal growth factor receptor-mutated non-small cell lung cancer.
SOLID TUMOR TRIALS

CYBRID-02 (BSK22-562)
Sponsor: Elephas Biosciences Corporation
Principal Investigator: Dr. David Hays
Observational basket trial to collect tissue to train and validate a live tumor diagnostic platform
ClinicalTrials.gov ID: NCT05520099

Pan-tumor MRD Study; FH-PrwS-07-002
Sponsor: Flatiron Health, Inc.
Principal Investigator: Dr. Andrew Briggler
Molecular Residual Disease in Solid Tumors: A Prospective Study to Collect Clinical Data and Biospecimens to Support Discovery and Development of Cancer Biomarkers
ClinicalTrials.gov ID: NCT06605404

NRG-CC012CD; Symon
Sponsor: NRG Oncology [Mercy Research]
Principal Investigator: Dr. Sam Makhoul
Managing Symptoms and Psychological Distress During Oral Anti-Cancer Treatment
ClinicalTrials.gov ID: NCT06279013
GASTROINTESTINAL (GI) CANCER TRIALS

RIVER-mPDAC; C3651021
Sponsor: Pfizer, Inc.
Principal Investigator: Dr. Ryan Hall
A Study to Learn About the Medicine Ponsegromab in Adults With Cancer of the Pancreas Which Has Spread and Caused Significant Body Weight Loss and Fatigue
BIOSPECIMEN TRIALS


iPROCESS
Sponsor: Bluestar Genomics & Iprocess Global Research Inc.
Principal Investigator: Dr. Kamal Patel
Collection and Distribution of Biofluids for Research Purposes Cancer Types Protocol

MT Group
Sponsor: The MT Group
Principal Investigator: Dr. Kamal Patel
Translational Medicine: Discovery and Evaluation of Biomarkers/Pharmacogenomics
for the Diagnosis and Personalized Management of Patients
RESEARCH NEWS AND EVENTS
Learn what research and clinical trials can mean for you from our Medical Director of Research, Dr. Sam Makhoul.
Watch this video to hear about the inspirational cancer journey of Carol Wadley and the role research played in her care.
ABSTRACTS AND ARTICLES BY CARTI PHYSICIANS
- Phase IIIb Safety and Efficacy of Intravenous Nepa for Prevention of Chemotherapy-Induced Nausea and Vomiting (CINV) in Patients with Breast Cancer Receiving Initial and Repeat Cycles of Anthracycline and Cyclophosphamide (AC) Chemotherapy. Kamal Patel, M.D., The Oncologist.
- Mortality and Causes of Death for Patients with Polycythemia Vera: Analysis of the REVEAL Prospective, Observational Study. Kamal Patel, M.D., American Society of Hematology.
- Demographics, Prior Therapies and Reasons for Cemiplimab Treatment: Prospective CemiplimAb-rwlc Survivorship and Epidemioogy (C.A.S.E.) Study in Patients with Advanced Cutaneous Squamous Cell Carcinoma. Rhonda Gentry, M.D.
- The Right to Try, Kamal Patel, M.D., The Healthcare Journal of Arkansas.

